Peter Lobner
The accident at Chernobyl Unit 4 occurred on 26 April 1986. A post-accident view of the Unit 4 reactor building is shown below.
Source: G.G. Pretzsch, et al. (2002)
A temporary “sarcophagus” was hastily erected around Unit 4 to provide some protection for the recovery workers and the public, to stabilize the damaged building and protect its interior from the effects of weather. Since November 2016, Unit 4 has been fully enclosed within the more substantial New Safe Confinement (NSC) building. You’ll find a good overview of the NSC at the Chernobyl Gallery website here: http://www.chernobylgallery.com/chernobyl-disaster/new-safe-confinement/
On 5 May 2021, Richard Stone, writing for Science magazine, reported online that, “Sensors are tracking a rising number of neutrons, a signal of fission, streaming from one inaccessible room, Anatolii Doroshenko of the Institute for Safety Problems of Nuclear Power Plants (ISPNPP) in Kyiv, Ukraine, reported last week during discussions about dismantling the reactor..….ever since its (the NSC) emplacement, neutron counts in most areas in the Shelter have been stable or are declining. But they began to edge up in a few spots, nearly doubling over 4 years in room 305/2, which contains tons of FCMs (fuel containing material) buried under debris.” Modeling by the ISPNPP suggests that the increasing neutron count rates may be related to the gradual drying of the FCMs. Other phenomena may be contributing, such as the observed long-term disintegration and change of consistency of some FCM formations in the rubble.
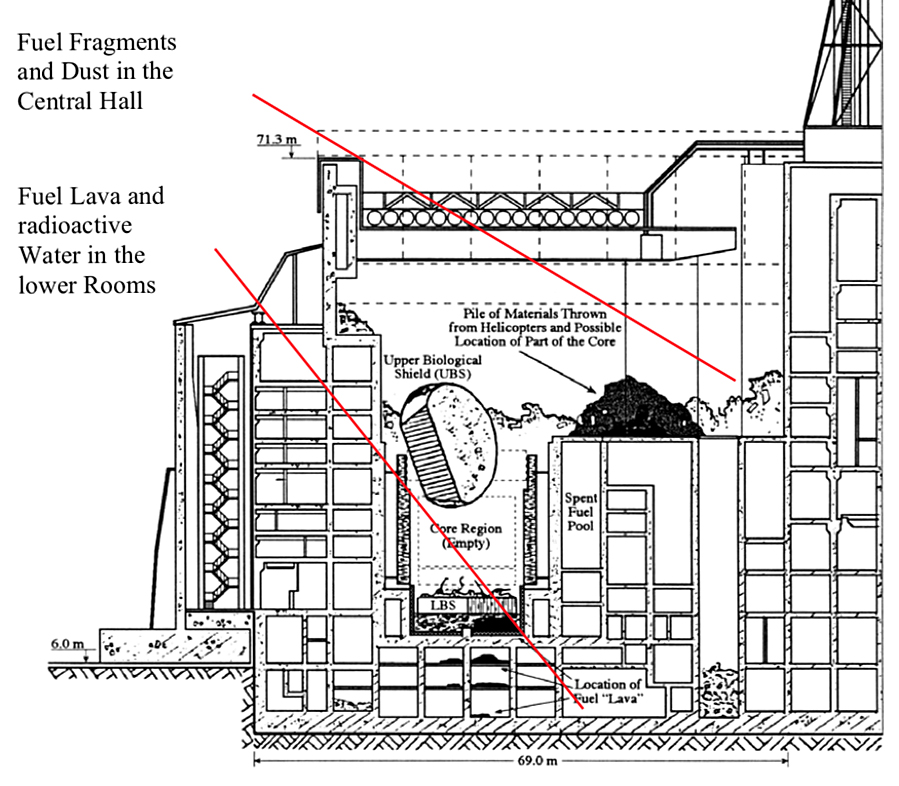
The ceiling of room 305/2 was directly under the Unit 4 reactor core. From the force of the accident, that ceiling was driven down by almost four meters.
The original inventory of uranium in the Unit 4 core was about 180 metric tons enriched to 3%. In a French-German study of the condition of the Chernobyl sarcophagus, authors G.G. Pretzsch, et al. reported that about 96% of the original nuclear fuel inventory remained inside the sarcophagus. The distribution was estimated as summarized in the following table. The authors estimated that about one-half of the total fuel mass was in Room 305/2.
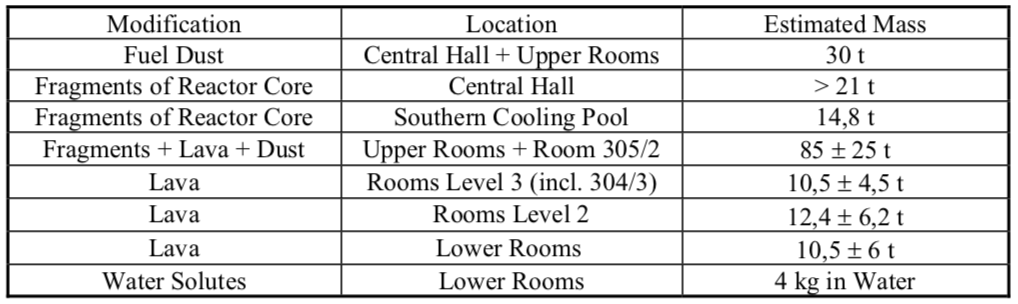
Chernobyl Unit 4. Source: G.G. Pretzsch, et al. (2002)
The condition of room 305/2 is described in considerable detail (in Russian) in the 1998 IAEA Report INIS-UA—062, “Room 305/2 Block 4 of the Chernobyl NPP: Its Condition, Assessment of the Amount of Fuel.” The room is a jumble of damaged building structural elements, reactor parts, and FCM in various forms, including “lava” flows.
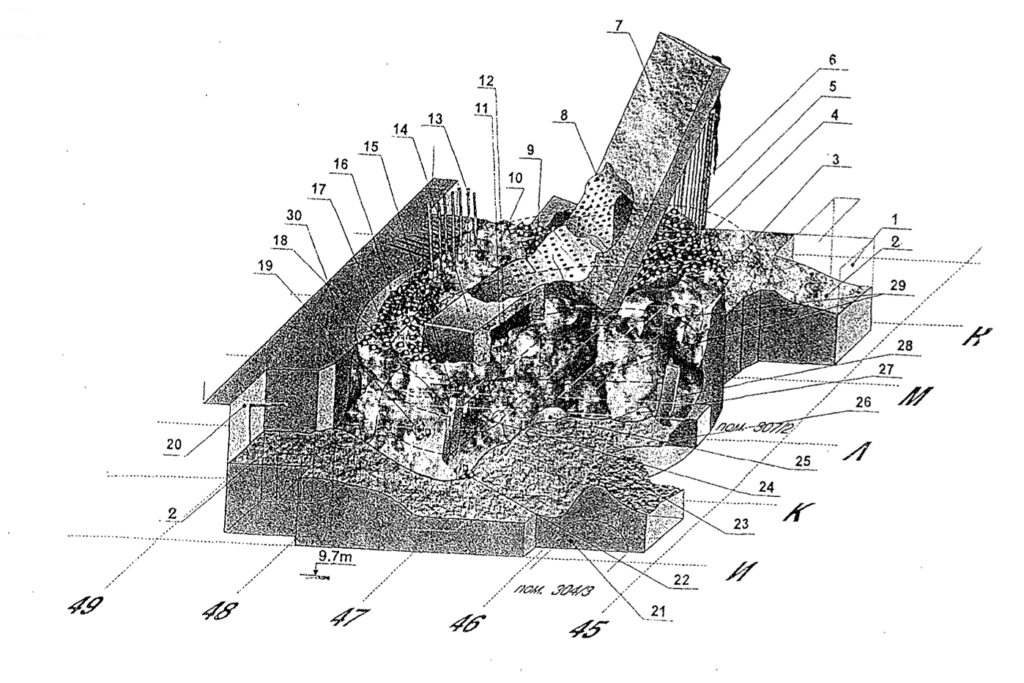
Source: A.A. Borovov, et al. (1998)
The authors reported on estimates developed using a variety of methods, as summarized in the following table, and concluded that the best estimate for room 305/2 was ≥ 60 metric tons of uranium.
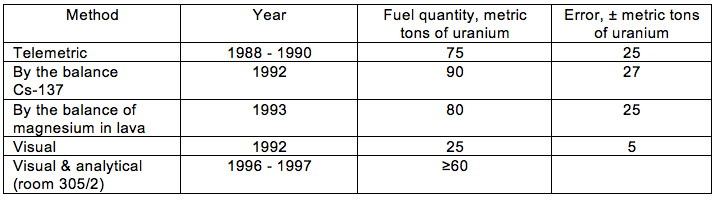
Source: A.A. Borovov, et al. (1998)
You’ll find my machine translation of this IAEA report to English, including the legend for the above figure, at the following link: https://lynceans.org/wp-content/uploads/2021/05/Chernobyl-Room-305_2-Assessment.pdf
Ukraine has long intended to remove the FCMs from the Unit 4 debris and store them in a geological repository. This plan remains under development, but now may have a new sense of urgency.
For more information
- Richard Stone, “‘It’s like the embers in a barbecue pit.’ Nuclear reactions are smoldering again at Chernobyl,” ScienceMag, 5 May 2021: https://www.sciencemag.org/news/2021/05/nuclear-reactions-reawaken-chernobyl-reactor?utm_campaign=news_daily_2021-05-05&et_rid=215579562&et_cid=3762851
- G.G. Pretzsch, et al., “The French-German Initiative for Chernobyl Sarcophagus Waste Management,” International Atomic Energy Agency, 2002: https://inis.iaea.org/collection/NCLCollectionStore/_Public/37/115/37115712.pdf?r=1&r=1
- A.A. Borovov, et al., “Room 305/2 Block 4 of the Chernobyl NPP: Its Condition, Assessment of the Amount of Fuel,” Report INIS-UA- -062 (in Russian), International Atomic Energy Agency, 1998: Summary only: https://inis.iaea.org/search/search.aspx?orig_q=RN:32020405 and direct link to the complete report: https://inis.iaea.org/collection/NCLCollectionStore/_Public/32/020/32020405.pdf?r=1




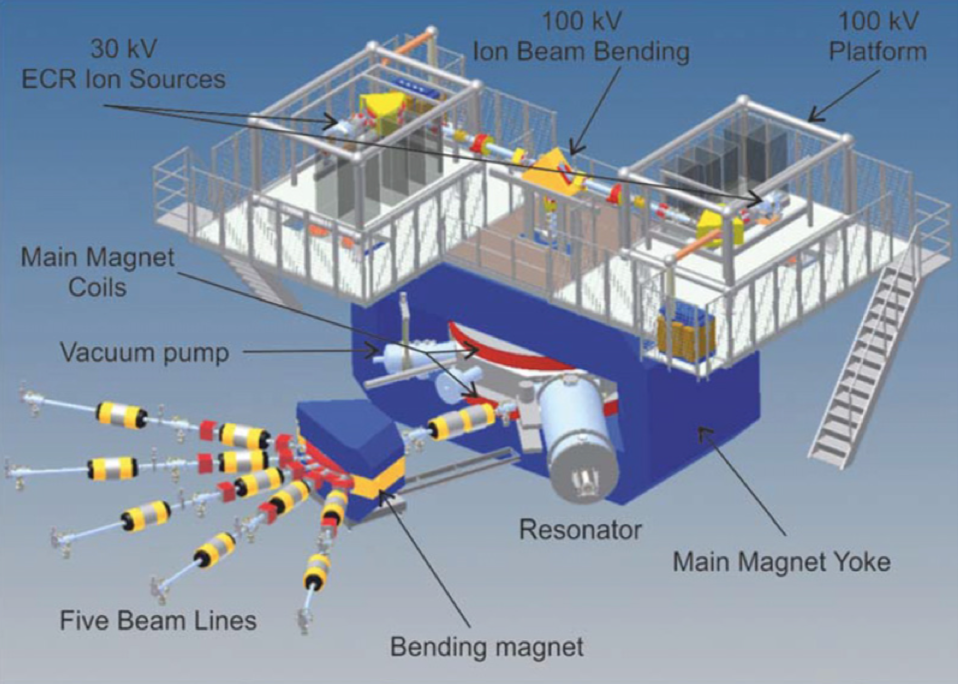

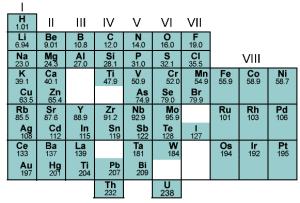

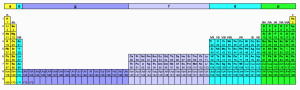
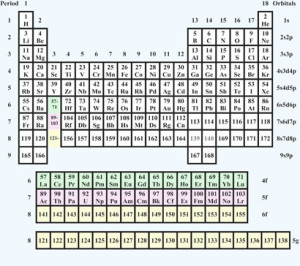
 The 1885 periodic table. Source: University of St. Andrews
The 1885 periodic table. Source: University of St. Andrews