Peter Lobner
In my 24 December 2016 post, I reported that the Qualcomm Tricorder XPrize committee had selected two teams to continue into the finals: Dynamical Biomarkers Group and Final Frontier Medical Devices. On 12 April 2017, the Qualcomm Tricorder XPrize committee announced the winners…and yes, there were two winners:
“Of the 300 teams that joined the pursuit of the Qualcomm Tricorder XPRIZE, Final Frontier Medical Devices and Dynamical Biomarkers Group were both announced winners at the Qualcomm Tricorder XPRIZE awards ceremony on April 12, 2017.
Final Frontier Medical Devices was announced the highest performing team and received $2.5M for their achievement and Dynamical Biomarkers Group received $1M for 2nd place. Both teams exceeded the competition requirements for user experience, nearly met the challenging audacious benchmarks for diagnosing the 13 disease states, and with their prototypes, have taken humanity one step closer to realizing Gene Roddenberry’s 23rd century sci-fi vision. XPRIZE congratulates Final Frontier Medical Devices and Dynamical Biomarkers Group on their amazing achievements.”
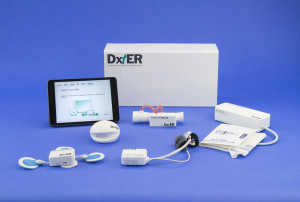
Learn more about Final Frontier Medical Devices and their winning tricorder named DxtER here:
http://tricorder.xprize.org/teams/final-frontier-medical-devices
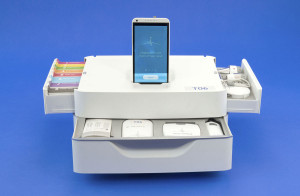
Learn more about Dynamical Biomarkers Group and their 2nd place tricorder system comprised of three modules here:
http://tricorder.xprize.org/teams/dynamical-biomarkers-group
OK, neither XPrize tricorder prototype looks like Dr. McCoy’s hand-held tricorder seen on Star Trek (the original series), but the automated diagnostic capabilities offered by the XPrize tricorder prototypes really are a giant leap forward in the development of tricorder technology for the real world. The Qualcomm Tricorder XPrize competition has been successful in making this happen on an accelerated schedule.
 McCoy and his tricorder. Source: Star Trek (the original series), Desilu Productions
McCoy and his tricorder. Source: Star Trek (the original series), Desilu Productions
Star Trek Tricorder replica. Source: Amazon.com