Peter Lobner
In my 17 December 2016 post, “Climate Change and Nuclear Power,” there is a chart that shows the results of a comparative life cycle greenhouse gas (GHG) analysis for 10 electric power-generating technologies. In that chart, it is clear how carbon dioxide capture and storage technologies can greatly reduce the GHG emissions from gas and coal generators.
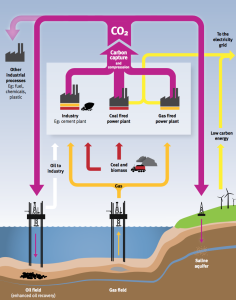
An overview of carbon dioxide capture and storage technology is presented in a December 2010 briefing paper issued by the London Imperial College. This paper includes the following process flow diagram showing the capture of CO2 from major sources, use or storage of CO2 underground, and use of CO2 as a feedstock in other industrial processes. Click on the graphic to enlarge.
You can download the London Imperial College briefing paper at the following link:
Here is a brief look at selected technologies being developed for underground storage (sequestration) and industrial utilization of CO2.
Store in basalt formations by making carbonate rock
Iceland generates about 85% of its electric power from renewable resources, primarily hydro and geothermal. Nonetheless, Reykjavik Energy initiated a project called CarbFix at their 303 MWe Hellisheidi geothermal power plant to control its rather modest CO2 emissions along with hydrogen sulfide and other gases found in geothermal steam.
 Hellisheidi geothermal power plant. Source: Power Technology
Hellisheidi geothermal power plant. Source: Power Technology
The process system collects the CO2 and other gases, dissolves the gas in large volumes of water, and injects the water into porous, basaltic rock 400 – 800 meters (1,312 – 2,624 feet) below the surface. In the deep rock strata, the CO2 undergoes chemical reactions with the naturally occurring calcium, magnesium and iron in the basalt, permanently immobilizing the CO2 as environmentally benign carbonates. There typically are large quantities of calcium, magnesium and iron in basalt, giving a basalt formation a large CO2 storage capacity.
The surprising aspect of this process is that the injected CO2 was turned into hard rock very rapidly. Researchers found that in two years, more that 95% of the CO2 injected into the basaltic formation had been turned into carbonate.
For more information, see the 9 June 2016 Washington Post article by Chris Mooney, “This Iceland plant just turned carbon dioxide into solid rock — and they did it super fast,” at the following link:
The author notes,
“The researchers are enthusiastic about their possible solution, although they caution that they are still in the process of scaling up to be able to handle anything approaching the enormous amounts of carbon dioxide that are being emitted around the globe — and that transporting carbon dioxide to locations featuring basalt, and injecting it in large volumes along with even bigger amounts of water, would be a complex affair.”
Basalt formations are common worldwide, making up about 10% of continental rock and most of the ocean floor. Iceland is about 90% basalt.
Detailed results of this Reykjavik Energy project are reported in a May 2016 paper by J.M. Matter, M. Stute, et al., “Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions,” which is available on the Research Gate website at the following link:
Similar findings were made in a separate pilot project in the U.S. conducted by Pacific Northwest National Laboratory and the Big Sky Carbon Sequestration Partnership. In this project, 1,000 tons of pressurized liquid CO2 were injected into a basalt formation in eastern Washington state in 2013. Samples taken two years later confirmed that the CO2 had been converted to carbonate minerals.
These results were published in a November 2016 paper by B. P McGrail, et al., “Field Validation of Supercritical CO2 Reactivity with Basalts.” The abstract and the paper are available at the following link:
http://pubs.acs.org/doi/pdf/10.1021/acs.estlett.6b00387
Store in fractures in deep crystalline rock
Lawrence Berkeley National Laboratory has established an initiative dubbed SubTER (Subsurface Technology and Engineering Research, Development and Demonstration Crosscut) to study how rocks fracture and to develop a predictive understanding of fracture control. A key facility is an observatory set up 1,478 meters (4,850 feet) below the surface in the former Homestake mine near Lead, South Dakota (note: Berkeley shares this mine with the neutrino and dark matter detectors of the Sanford Underground Research Facility). The results of the Berkeley effort are expected to be applicable both to energy production and waste storage strategies, including carbon capture and sequestration.
You can read more about this Berkeley project in the article, “Underground Science: Berkeley Lab Digs Deep For Clean Energy Solutions,” on the Global Energy World website at the following link:
Make ethanol
Researchers at the Department of Energy’s Oak Ridge National Laboratory (ORNL) have defined an efficient electrochemical process for converting CO2 into ethanol. While direct electrochemical conversion of CO2 to useful products has been studied for several decades, the yields of most reactions have been very low (single-digit percentages) and some required expensive catalysts.
Key points about the new process developed by ORNL are:
- The electro-reduction process occurs in CO2 saturated water at ambient temperature and pressure with modest electrical requirements
- The nanotechnology catalyst is made from inexpensive materials: carbon nanospike (CNS) electrode with electro-nucleated copper nanoparticles (Cu/CNS). The Cu/CNS catalyst is unusual because it primarily produces ethanol.
- Process yield (conversion efficiency from CO2 to ethanol) is high: about 63%
- The process can be scaled up.
- A process like this could be used in an energy storage / conversion system that consumes extra electricity when it’s available and produces / stores ethanol for later use.
You can read more on this process in the 19 October 2016 article, “Scientists just accidentally discovered a process that turns CO2 directly into ethanol,” on the Science Alert website at the following link
The full paper is available on the Chemistry Select website at the following link:
http://onlinelibrary.wiley.com/doi/10.1002/slct.201601169/full